Abstract
Introduction: Mercaptopurine (MP) is one of the main chemotherapeutics for acute lymphoblastic leukemia (ALL), and constant MP dose titration is essential to maintain steady drug exposure, while minimizing myelosuppression. Throughout previous studies, thiopurine methyltransferase (TPMT) is proved to be one of the most responsible genes in the pharmacogenetics of MP. However, most of East Asian patients still show sensitivity to MP after adjusting for TPMT variants, suggesting the existence of other yet unknown factors that are related to MP sensitivity. In this study, the genetic factors affecting the toxicity of MP were investigated in Korean children with ALL.
Methods: Two-stage pharmacogenetic analyses were performed to identify genetic determinants of MP-related neutropenia in Korean pediatric ALL patients who received maintenance chemotherapy including daily MP. A total of 287 patients with ALL who were diagnosed and treated at the Seoul National University Children's Hospital and Asan medical center were included. First, targeted sequencing was carried out using a novel gene panel of 147 pharmacogenetics related genes and 8 SNPs in 44 patients who received less than 25% of initial dose during maintenance therapy due to MP intolerance. Next, significant genes identified in the first analysis were re-sequenced in a total of 287 patients. The relationship between mutation of each gene identified through sequencing and toxicity or dose of MP were analyzed.
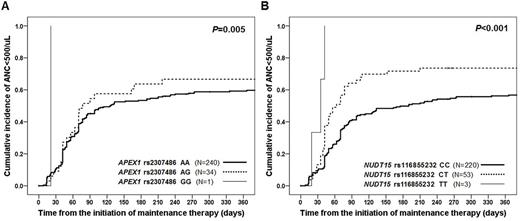
Results: As a result of the first stage analysis, 4 functionally interesting variants other than NUDT15 were selected (ABCC4, APEX1 , CYP1A1 ,and CYP4F2). Including these 4 variants, total 23 variants in 12 genes potentially linked to MP adverse reactions were selected as final candidates for subsequent analysis in 287 patients. The distribution of variant alleles were NUDT15 rs116855232 (20.7%), APEX1 rs2307486 (13.0%), ABCC4 rs2274407 (36.2%), ABCC4 rs3765534 (15.4%), ABCC4 rs11568658 (26.2%), CYP4F2 rs2108622 (54.5%), CYP1A1 rs4646422 (30.6%), SLCO1B1 rs11045879 (52.1%), SLCOB1 rs4149056 (27.0%), ITPA rs1127354 (25.6%), ITPA rs7270101 (0%), MTHFR rs1801131 (28.2%), MTHFR rs1801133 (68.3%), MTHFR rs1901133 (54.1%), GRIA1 rs4958351 (2.4%), MOCOS rs594445 (50.3%), PACSIN2 rs2413739 (9.4%), BAG3 rs78439745 (8.4%) and TPMT (* 1 / * 3C and *6, 3.1%). Grade 4 neutropenia (neutrophil<500/㎕) was observed in 177 patients (61.7%) during the administration of MP. When frequency of neutropenia during MP administration was crosstabulated with variant frequencies, the risk of neutropenia was 4.64 times higher in patients with T allele in ABCC4 rs3765534 (95% CI; 1.4 to 20.7, P=0.013). The cumulative incidence of neutropenia was significantly higher in patients with variant forms of APEX1 rs2307486 (AA:AG:GG=59.2%:66.7%:100%, P=0.005) (Figure 1). The cumulative incidence of neutropenia was also significantly increased in patients with NUDT15 rs116855232 mutations (CC:CT:TT=56.2%:73.6%:100%, P <0.001). According to the Cox regression analysis regarding neutropenia, GG genotype in APEX1 rs2307486 conferred a multivariate hazard ratio of 12.03 (95% CI; 1.4 to 101.2, P=0.022), and TT genotype in NUDT15 rs116855232 showed a hazard ratio of 11.42 (95% CI; 3.35 to 38.97, P <0.001). Comparing the average doses of MP used in the study, the T allele type of NUDT15 rs116855232 was significantly associated with a lower tolerated dose of MP as 61.7, 44.6, 20.1% of initially planned doses for CC, CT, and TT genotypes (P <0.001). However, MP doses by TPMT mutations were not significantly different in this study.
Conclusion: This study revealed that genetic variations APEX1 increases the cumulative incidence of neutropenia associated with MP, and the variant allele in NUDT15 was related to MP intolerance. APEX1 and NUDT15 both contribute to cell protection from DNA damage or misincorporation, so alleles that impair the function of either gene may affect MP sensitivities, thereby inducing MP-related neutropenia. In Korean ALL patients, toxicity and tolerance of MP were more affected by APEX1 and NUDT15 than by TPMT, which has a lower variant allele frequency in Asian.
Figure 1. Estimated cumulative incidence of mercaptopurine-related neutropenia according to the (A) APEX1 rs2307486 and (B) NUDT15 rs116855232 genotypes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal